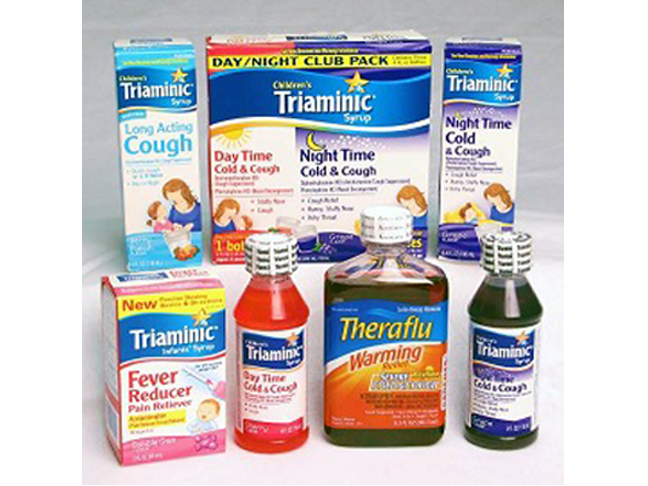
What Do I Need to Know about This Recall?
This recall involves several Triaminic® Syrups and Theraflu Warming Relief® Syrups that were manufactured prior to December 31, 2011. The recalled products include the following:
- Triaminic® Cough and Sore Throat, 4 oz.
- Triaminic® Chest and Nasal Congestion, 4 oz.
- Triaminic® Cold and Allergy, 4 oz.
- Theraflu® Warming Relief Cold and Chest Congestion, 8.3 oz.
- Theraflu® Warming Relief Sinus and Cold, 8.3 oz.
- Theraflu® Daytime/Nighttime Severe Cold & Cough Value Pack
- Theraflu® Nighttime Warming Relief Severe Cold and Cough, 8.3 oz.
- Theraflu® Daytime Warming Relief Severe Cold and Cough, 8.3 oz.
- Theraflu ® Warming Relief Flu and Sore Throat, 8.3 oz.
- Theraflu ® Warming Relief Flu and Sore Throat, 8.3 oz.
- Triaminic® Dye-free Long Acting Cough, 4 oz.
- Triaminic® Daytime/Nighttime Cold & Cough Club Pack
- Triaminic® Daytime/Nighttime Cold & Cough Combo Pack
- Triaminic® Day Time Cold and Cough, 4 oz.
- Theraflu ® Warming Relief Flu and Sore Throat, 8.3 oz.
- Triaminic® Night Time Cold and Cough, 4 oz.
- Triaminic® Multi Symptom Fever, 4 oz.
- Triaminic® Night Time Cold and Cough, 3.4 oz.
- Triaminic® Fever Reducer Pain Reliever Infant Grape, 2 oz.
- Triaminic® Multi Symptom Fever, 3.4 oz.
- Triaminic® Fever Reducer Pain Reliever Grape, 4 oz.
- Triaminic® Fever Reducer Pain Reliever Infant Bubblegum, 2 oz.
- Triaminic® Fever Reducer Pain Reliever Bubblegum, 4 oz.
Check your bottle to view the LOT and NDC numbers to determine if you are in possession of one of the recalled products. A full list of recalled products with their LOT and NDC numbes can be seen here.
- The LOT number is located on the bottom panel of the box and on the left side of the label on the bottle; click here to view an example.
- The NDC number is located on the upper right corner of the front panel of the box for Triaminic®, and the upper left corner of the front of the bottle for Theraflu®. Please click here to view an example.
These over-the-counter cough, cold and flu medications have been sold at drugstores nationwide and throughout Canada between May 2010 and December 2011
What are the Risks?
The child-resistant features of the bottle caps may fail, enabling children to be remove the caps. These products contain acetaminophen and diphenhydramine which are required by the Poison Prevention Packaging Act to be sealed with child-resistant packaging. Those who ingest unregulated quantities of these syrups may experience serious adverse health effects.
At this time Novartis has received 12 reports of children unscrewing the locked caps and four reports of children ingesting the product, one of whom required medical attention.
What Should I Do if I Purchased One of These Syrups?
Stop using this product immediately.
You can receive reimbursement by filling out the appropriate form:
Please follow the specific instructions on each form to ensure that your request gets processed properly.
Contact Novartis for additional information on receiving your refund via email at [email protected] or call 866-553-6742, Monday through Saturday, 8AM – 12AM Eastern Standard Time.
Additional Resources
See the official CPSC recall here.
See the official Health Canada recall here.
See the Norvartis recall here.
See FAQS here.